In vitro and additional techniques
In vitro fertilisation (IVF) is a method that involves the fertilisation of an egg outside the woman’s body.
In vitro fertilisation (IVF) is a method that involves the fertilisation of an egg outside the woman’s body.
What is in vitro fertilisation?
What is in vitro fertilisation?
In vitro fertilisation (IVF) involves the fertilisation of an egg obtained from the woman with her partner’s sperm outside the woman’s body.
This method consists of a few stages. Once a thorough history has been taken, the patients’ history has been analysed and the necessary tests have been performed, hormonal stimulation treatment is initiated. The woman is administered hormonal medication in order to obtain more than one egg cell. The collected reproductive cells are purified and then combined together under laboratory conditions. The resulting embryos are cultured under special conditions and transferred into the uterine cavity.
Diagnostics
Required testing
Female partner
Female partner
Male partner
Male partner
In order to carry out the in vitro fertilisation procedure, it is necessary to adequately prepare the cycle, perform the above tests, and complete the necessary documentation by both the female and male partner.
Treatment
Course of in vitro procedure
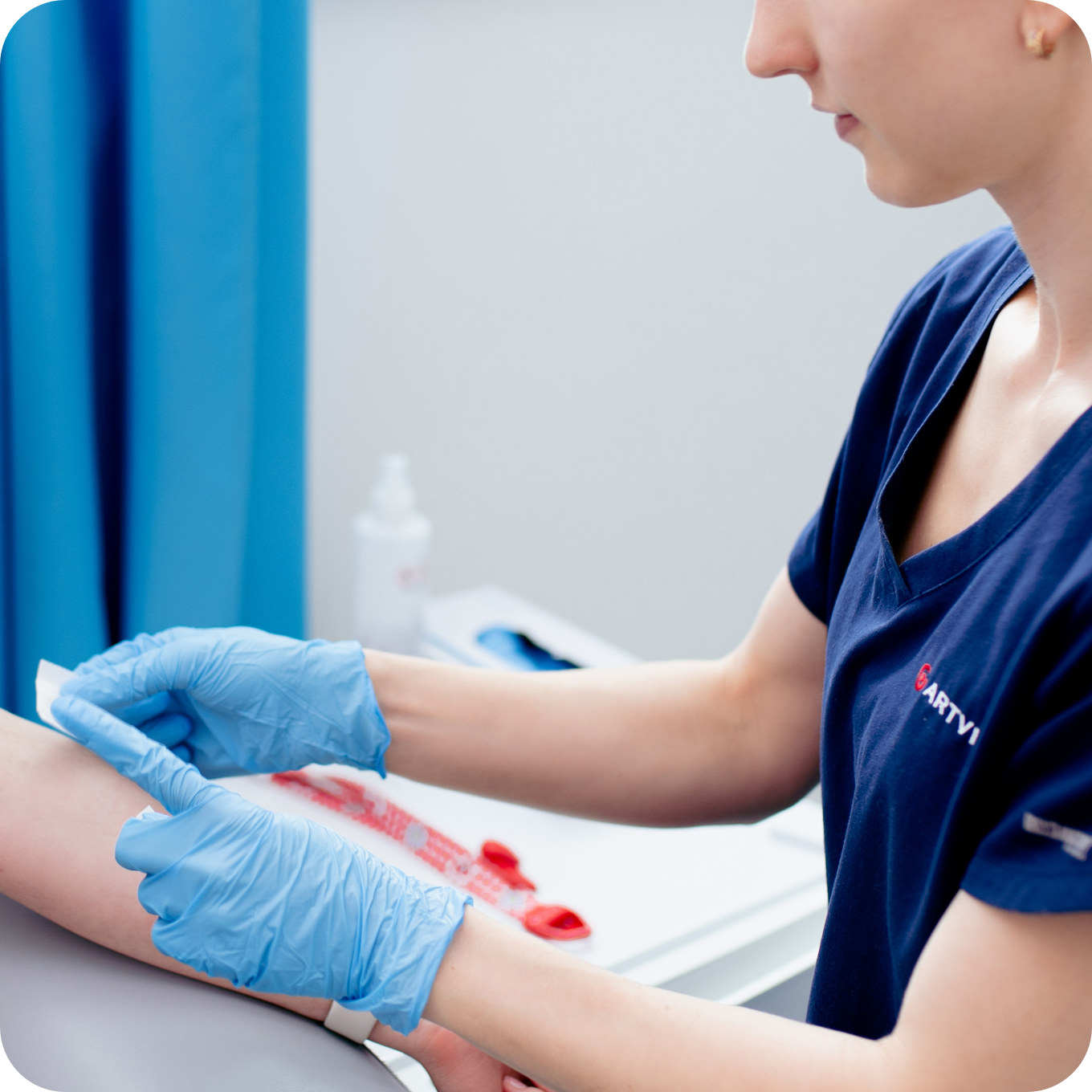

Stage I
Carrying out the necessary examinations
Results of the necessary tests, listed above, are required to qualify patients to participate in the IVF programme.

Stage II
Hormonal stimulation and monitoring of the patient’s cycle
Hormonal stimulation of the ovaries aims to achieve multiple ovulation, i.e. the growth of ovarian follicles containing egg cells – usually several to a dozen follicles are formed during stimulation.
The attending physician selects the appropriate stimulation method for each patient, considering factors such as age, body weight, ultrasound findings (number of antral follicles), and serum levels of FSH and AMH, among other parameters.
The course of stimulation is monitored by the attending physician on the basis of ultrasound examinations and assessment of serum concentrations of oestradiol and progesterone (on average 3-4 visits during stimulation).

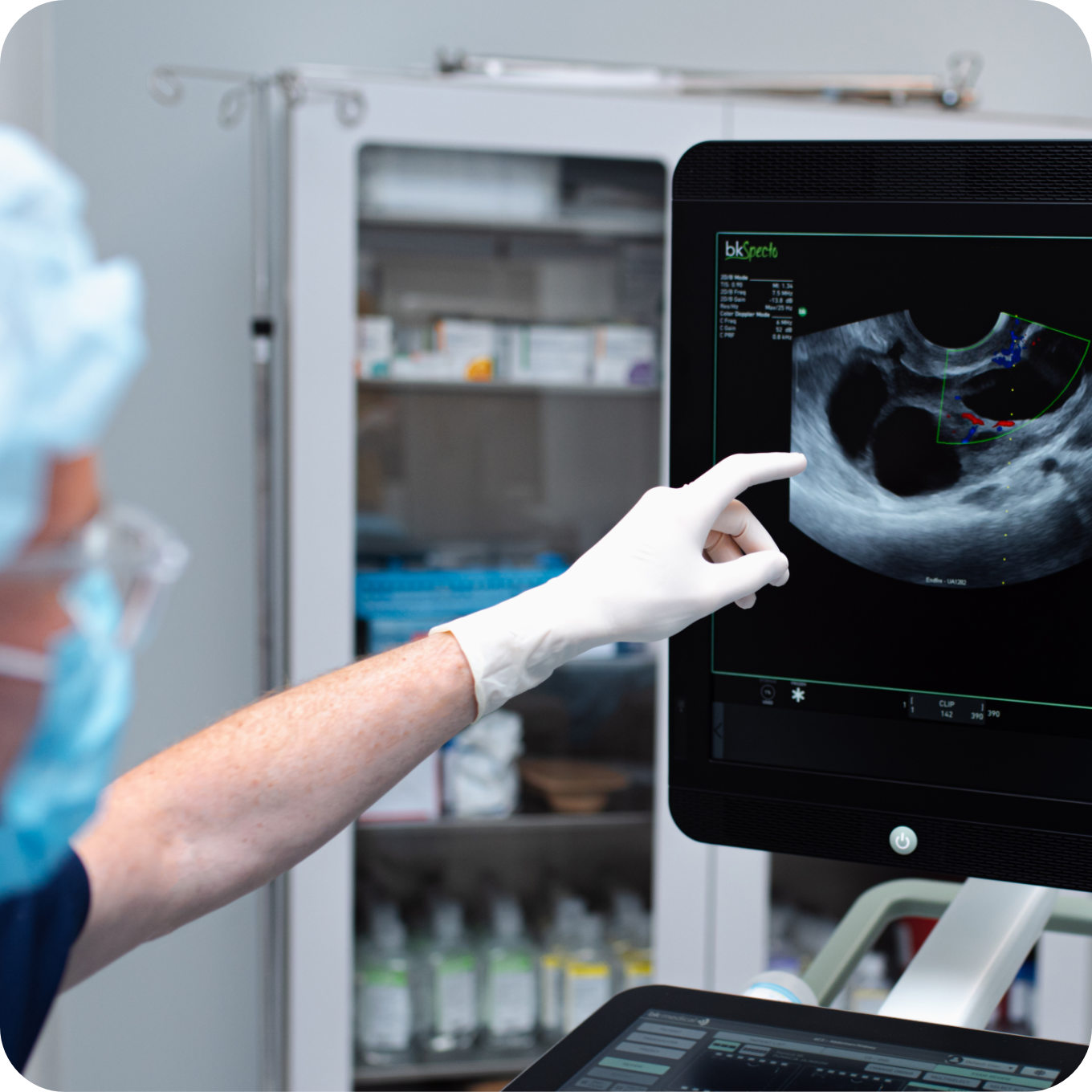

Stage III
Ovarian puncture
Ovarian puncture consists in collecting egg cells (female gametes) for in vitro fertilisation procedure. Once the follicles have reached an appropriate size, they are punctured under ultrasound guidance through the vaginal vault and the egg cells are retrieved. The procedure is carried out under short-term general anaesthesia.
The ovarian puncture is preceded by a consultation with the anaesthetist, during which he/she becomes acquainted with the patient’s condition. During the procedure, the patient is under the constant care of an anaesthetist who watches over her vital functions during which time the gynaecologist painlessly retrieves the egg cells.

Stage IV
In vitro fertilisation
It involves the fusion of gametes outside the woman’s body (in vitro) to produce an embryo.
The egg cells obtained during ovarian puncture are stored in appropriate conditions in a special incubator. Semen is donated by the man through masturbation, on the day of the puncture.
Once the ova have been collected, the embryologist combines the partner’s specially prepared sperm with the cells taken from the female partner. After 18 hours, it is assessed under the microscope whether fertilisation, i.e. the union of egg and sperm cells, has taken place.
The following day, the embryologist informs the patient by telephone of the results.



Stage V
Embryo culture
In the days following fertilisation, embryos are cultured and monitored in special incubators. During this phase of embryo development, the embryo undergoes numerous transformations, from the zygote stage through multi-blastomere stages, until it reaches the blastocyst stage on day 5, which is the optimal time for transfer into the uterine cavity.
We use an incubator fitted with a Time-Lapse system, which, through a built-in camera, allows us to continuously monitor the development of the embryos without removing them. This ensures that the embryos are in optimal, constant growing conditions at all times.
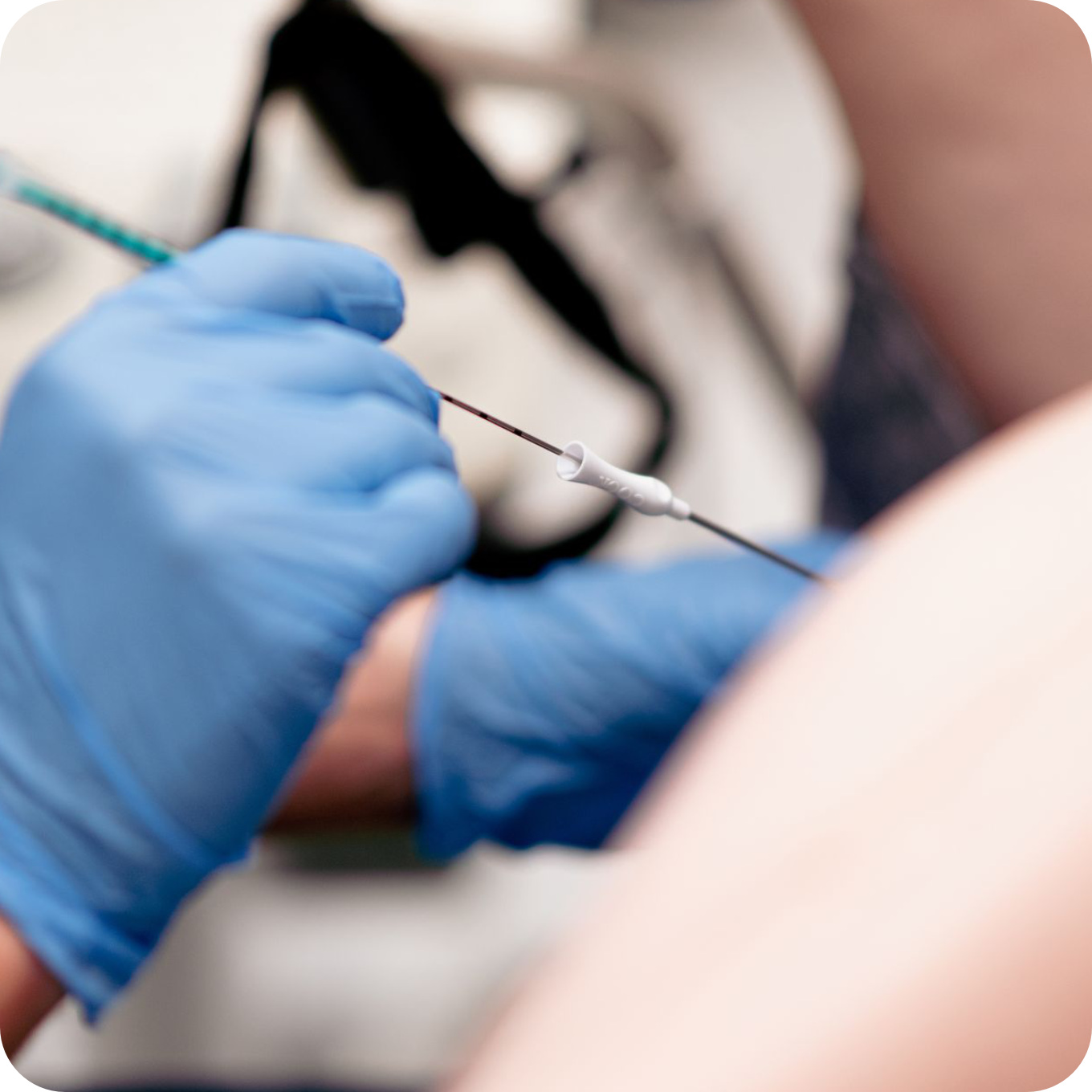
Stage VI
Embryo transfer
The developing embryos are inserted through a special catheter through the cervix into the uterine cavity, where they implant.
The procedure is painless and does not require anaesthesia. It is carried out with full bladder.

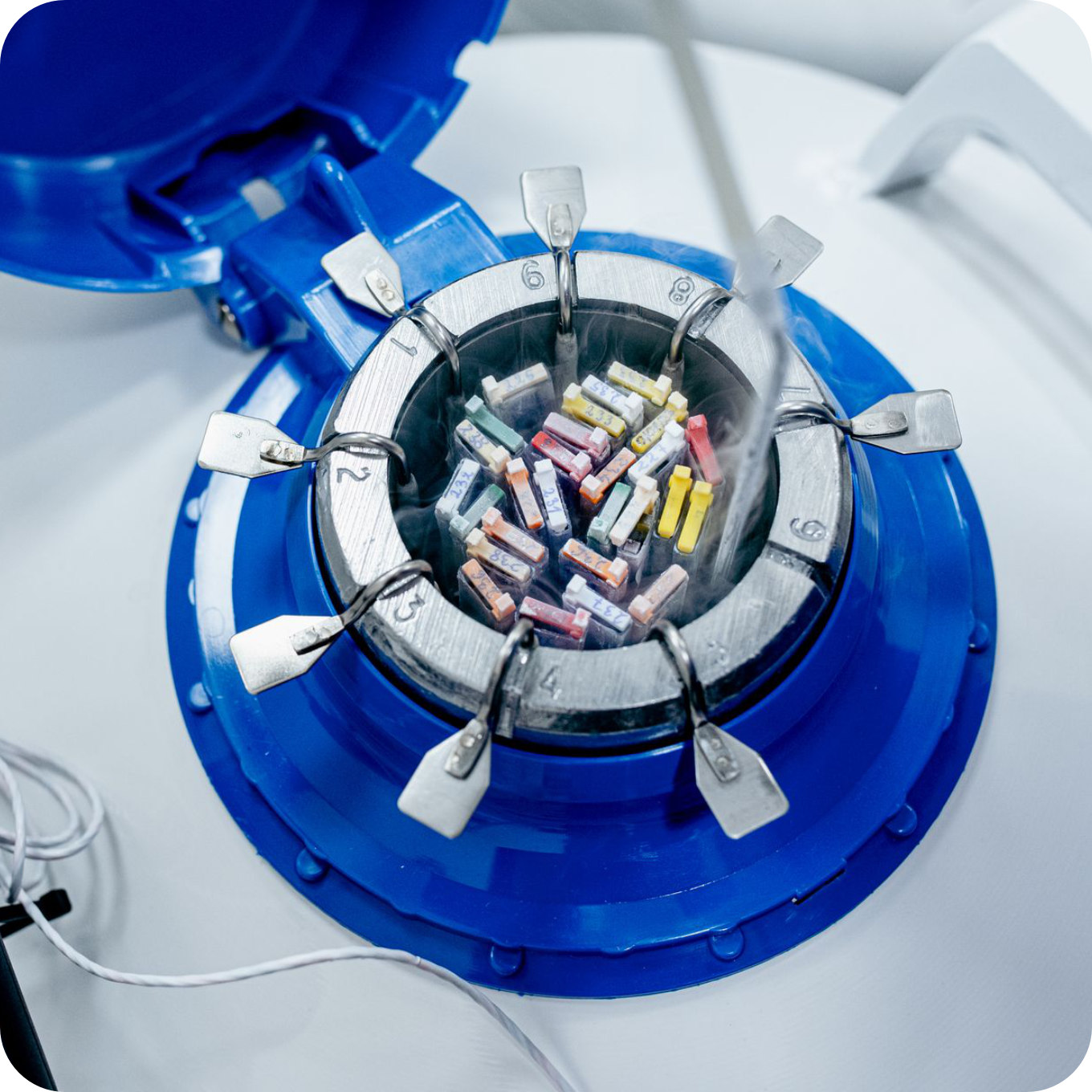

Stage VII
Freezing of other embryos
During the transfer, one embryo is usually administered into the uterine cavity. Only in justified cases (such as poor embryo quality, previous transfer failures, patient’s age above 38 years) is it acceptable to inject a maximum of two embryos. The remaining embryos retrieved are frozen.
Embryo freezing is carried out using the vitrification technique – a very safe and effective method that allows surplus embryos to be preserved for subsequent transfers. This prevents patients from being subjected to further stimulation and ovarian punctures. Transfers of thawed embryos can be performed in natural cycles or after a short hormonal preparation (in an artificial cycle or with ovulation induction).

Good to know
All you should
know about this method

The in vitro fertilisation procedure is intended for couples who have tried to have a child with no success. It is used as a last resort if other methods, such as cycle monitoring or insemination, fail or cannot be recommended to the couple for health reasons.
In vitro is a procedure and treatment method available to couples with diagnosed infertility. Indications for IVF are:
- obstructed or missing fallopian tubes
- severe endometriosis
- failure to induce ovulation
- hormonal disorders
- ovarian dysfunction
- reduced ovarian reserve
- anatomical defects
- poor semen parameters
- idiopathic infertility
Contraindications to IVF include the woman’s overall poor health, certain cancers, autoimmune and chronic diseases, precluding hormonal stimulation.
The in vitro fertilisation procedure is a complex process that requires good preparation. On top of doing the vital tests required for the IVF/ICSI procedure, you should ensure that you adopt a healthy lifestyle, eat a balanced diet and exercise regularly.
Preparation for couples:
Sexual abstinence must be strictly observed while preparing for the in vitro fertilisation procedure.
Patients with cardiovascular, oncological, neurological, or other conditions are required to inform their attending physician about the commencement of preparations for in vitro fertilisation and to obtain a certificate confirming no contraindications for infertility treatment.
Excess weight in both men and women can reduce the effectiveness of natural and assisted pregnancy attempts, so it is important to achieve a healthy body weight before beginning assisted reproduction procedures. You should start a proper diet and supplementation at least 3 months prior to the IVF procedure.
Preparation for women:
- intake of folic acid – folic acid preparations (Pregna Plus, Feminatal Metafolin 800) 1 tbl/day, preferably for 3 months before the scheduled treatment
-
intake of vit.D3 between September and June at a dose of 2,000 IU/d, preferably before prior testing of vit.Serum vitamin D3 level
- protein-rich diet
- avoidance of spicy foods that irritate the intestines
- do not take laxatives; if constipation occurs, we recommend glycerine suppositories, flaxseed or, as a last resort, a lactulose syrup.
- sufficient hydration ( a minimum of 1.5-2 litres of water/day)
Preparation for men:
Two to seven days of sexual abstinence are required prior to sperm donation for in vitro fertilisation. You should maintain a minimum of two days of alcohol abstinence and also limit excessive physical exertion.
The patient should also not be on antibiotics. The quality of semen is also adversely affected by increased body temperature, including fever. In such cases, please notify the attending physician.
If worrying symptoms appear (severe abdominal pain, pain in the clavicular region, fever, fainting, bleeding from the genital tract), the patient should immediately contact the clinic staff, or the nearest gynaecology and obstetrics ward.
Ovarian hyperstimulation syndrome
Some patients may develop hyperstimulation syndrome during the course of hormonal stimulation, which is manifested by an excessive response to stimulant medication. As a result of this response, numerous cysts form in the ovaries, fluid seeps into the abdominal cavity and urine output decreases. This may be accompanied by pain and enlargement of the abdominal girth, nausea, vomiting, diarrhoea and, in more severe cases, dyspnoea and chest pain. Very rarely, hyperstimulation syndrome can be accompanied by thrombosis and pulmonary embolism.
Severe hyperstimulation syndrome may require treatment, including hospitalisation, or intravenous fluid and albumin administration.
Symptoms usually resolve around the 6th to 8th week of pregnancy.
If there is a high risk of developing hyperstimulation syndrome, the doctor monitoring the course of stimulation may suggest the following treatment:
- discontinuation of stimulation without puncture and cell retrieval (the risk of developing hyperstimulation syndrome is then minimal)
- discontinuation of treatment at a later stage – after puncture and fertilisation of the oocytes, all embryos thus obtained are frozen without transfer. Embryos are transferred in later cycles after thawing.
If pregnancy does not occur, the occurrence of hyperstimulation syndrome is unlikely. The risk of hyperstimulation syndrome does not invalidate the patient’s chances of achieving a pregnancy in subsequent cycles. However, the appearance of symptoms of hyperstimulation syndrome in early pregnancy increases the risk of miscarriage.
Infections and bleeding
It is exceptionally rare to have complications after the procedure in the form of infection or abdominal bleeding. Very seldom such a complication requires hospital treatment.
Foetus defects
There was no significantly higher incidence of foetal abnormalities following assisted reproduction technology methods.
The incidence of defects is minimally higher than in the general population, where the risk is about 4-5%, which is probably due to infertility itself rather than its treatment methods. According to various sources, the incidence of foetal abnormalities following assisted reproduction techniques is approximately 7-9%.
There is a slight increase in the incidence of chromosomal abnormalities (abnormal number of chromosomes) after the ICSI method and it is approximately 1.56%.The prevalence of these disorders in the general population is approximately 0.5%.This may be related to the higher incidence of abnormal chromosome number in the sperm of infertile men.
As recommended by the Polish Society of Gynaecologists, all pregnant women (not only after infertility treatment) should have prenatal tests, including, in the first instance, the so-called genetic ultrasound and the dual test at 11th to 13th week of pregnancy. The result of this test determines whether or not further invasive diagnostic tests such as amniocentesis are necessary.
No response to stimulation
In some patients, especially those over 37 years of age, with elevated FSH levels, reduced AMH levels, there may be no or very little follicle growth (1-2 follicles). This group of patients is referred to in the literature as ‘poor responders’. In such a situation, therapy may have to be discontinued (due to its futility). In such circumstances, the attending physician may suggest the following to the patient:
- change of stimulation protocol in the next cycle
- in vitro fertilisation in the natural cycle
- use of the egg cell or embryo adoption programme
Long-term complications
Pregnancy following treatment with assisted reproductive technology is a pregnancy at increased obstetric risk due to the increased risk of miscarriage, preterm birth (usually associated with the presence of multiple pregnancies), prematurity and low birth weight (resulting from reduced intrauterine growth of the foetus).
More about this procedure
Types of in vitro fertilisation
Classic IVF
It requires the cells collected during the puncture to be placed in a culture dish and then the previously prepared semen to be added. Fertilisation occurs spontaneously. This method is most commonly used in cases of infertility due to an ovarian factor in the absence of abnormal sperm parameters.
ICSI method
It involves the insertion of sperm directly into the egg using a very thin glass micropipette. This method is used if the sperm count in a man’s semen is too low or its motility is abnormal, or if past attempts at in vitro fertilisation had failed (no fertilisation) or if the number of cells retrieved is low (<5), intracytoplasmic sperm injection is used.
pICSI method
(phisiological ICSI) – a variation of the classic ICSI method, augmented with additional sperm selection using its ability to bind to hyaluronic acid.
According to the method of sperm-to-oocyte introduction, there are several varieties of in vitro fertilisation. The attending physician and the embryology team decide on the method, taking into account the couple’’s specific clinical situation.
The cumulative post-IVF pregnancy rate is:
The cumulative post-IVF pregnancy rate is:
up to 74%*
There are a number of factors that affect the outcome of treatment, these include the woman’s age, body weight, semen quality and the presence of other co-morbidities. The positive outcome of IVF infertility treatment is also impacted by the patient’s personal response to hormonal stimulation.
*Cumulative pregnancy rate per cycle of treatment at our clinic in 2022

In vitro fertilisation
Additional techniques
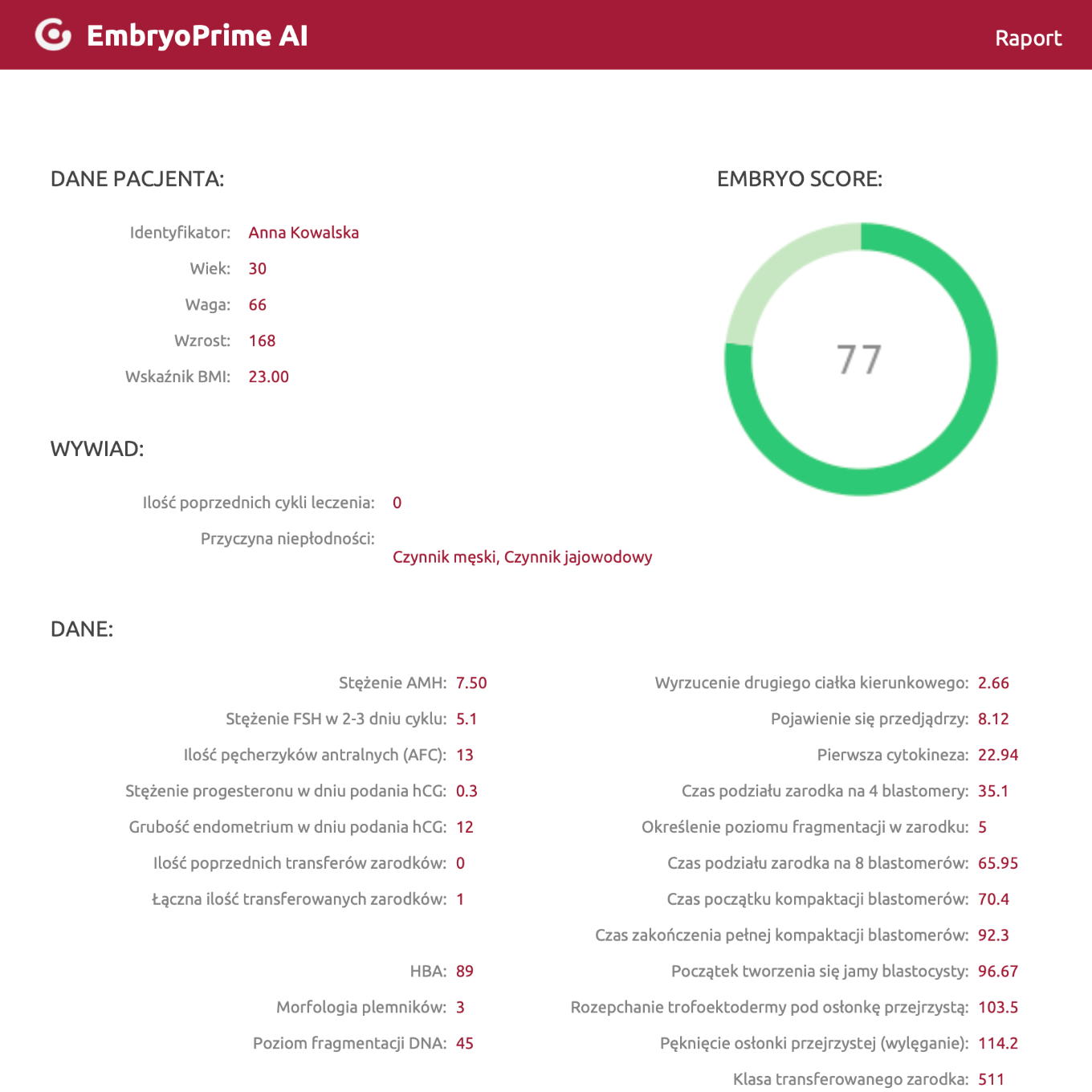

EmbryoPrime AI support
We use an AI-based tool we developed to help embryologists select the embryo for transfer that offers patients the best chance of achieving a healthy pregnancy.
We developed the self-learning algorithm behind the EmbryoPrime AI tool based on thousands of clinical and embryological data points collected over several years of work.
The embryologist enters the patient’s data, weight and height and the cause of infertility, hormone levels and embryo culture data, etc. After being calculated by the algorithm based on statistical data, we obtain information about the chances of the embryo resulting in a healthy pregnancy.
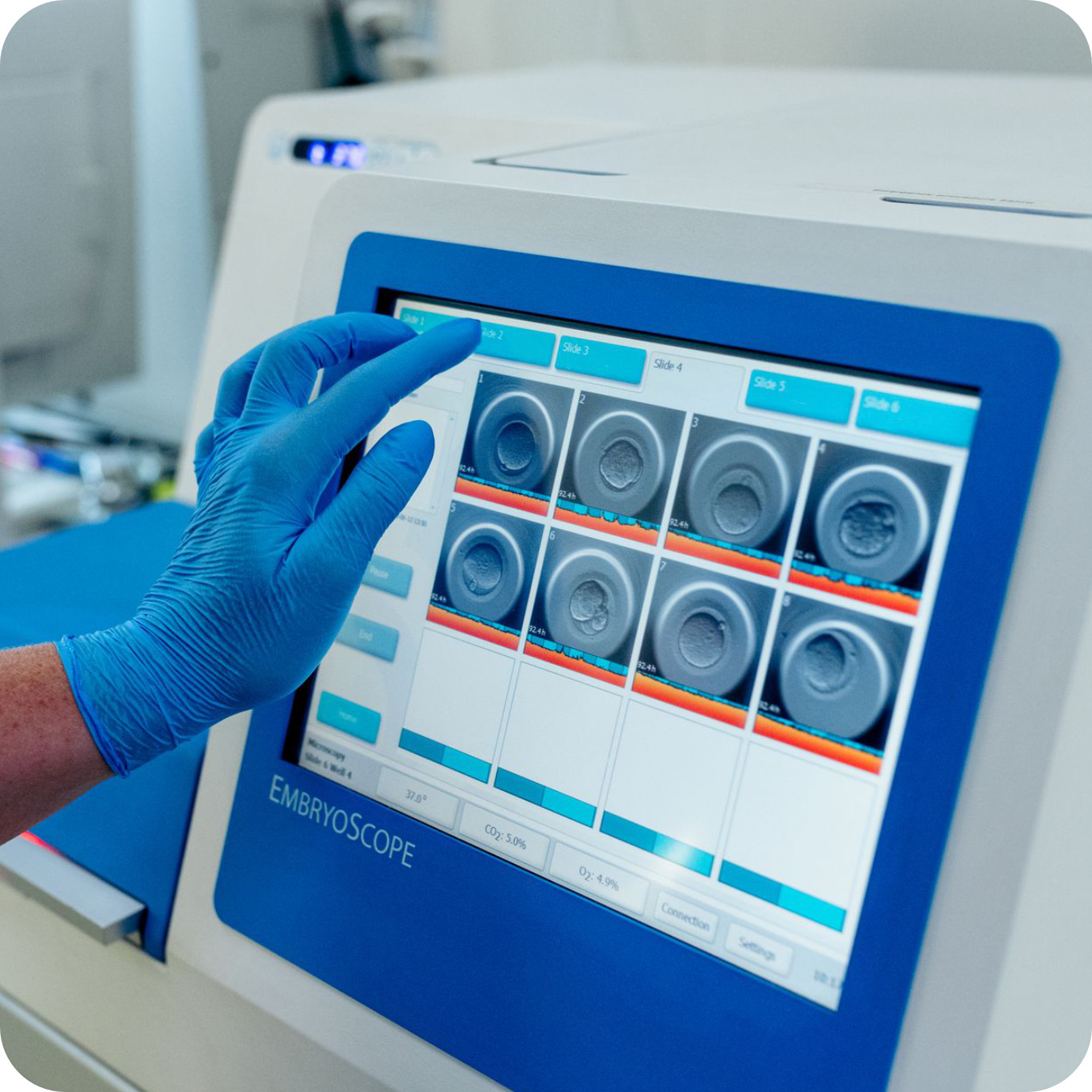
Time-Lapse System
Traditional embryo culture methods face the major disadvantage of requiring repeated removal of embryos from the incubator so that they can be assessed. Even though all precautions are taken, temporary fluctuations in the temperature of the culture media and changes in gas concentrations in the incubator are inevitable. This is likely to have a major impact on the course of their development.
The time-lapse system built into the incubator allows for continuous embryonic development monitoring, with no need to remove the embryos from the incubator. The embryos are kept under constant culture conditions at all times, so the risk of adverse events occurring due to modification of conditions is restricted to a minimum. Using its internal cameras, the system provides information on the timing of cell divisions and the dynamics of morphological changes, allowing us to select the most promising embryo for transfer.



FertileChip
This method consists of reproducing the sperm’s behaviour during its natural migration through the female reproductive system. Microfluidic technology allows the sperm to travel through a special porous membrane. The selected sperm has better motility, morphology and DNA integrity, as compared to traditional sperm separation techniques.
The FertileChip method is recommended for cases involving reduced semen parameters, abnormal sperm DNA fragmentation, elevated oxidative stress levels in sperm, unsuccessful IVF attempts (such as a low percentage of developing embryos, poor embryo quality, or lack of implantation), and recurrent miscarriages.
EmbryoGlue
A special medium designed for embryo transfer. It is used to improve implantation rates and thus boost the rate of resulting pregnancies. It closely mirrors the conditions in the uterus at the time of implantation thanks to the hyaluronate present in the formulation.
EmbryoGlue is recommended for transfers in patients over 35 years of age, patients with previous unsuccessful implantation attempts or patients with idiopathic infertility.


Assisted hatching (AH)
In plain language: assisted hatching requires a small incision in the zona pellucida of the embryo just before its transfer. Assisted hatching may improve the chances of successful embryo implantation if the zona pellucida is excessively thick or hard (e.g., after freezing), which can prevent the embryo from hatching naturally.
Assisted hatching is performed for patients aged 37 and over, couples who have experienced two or more unsuccessful IVF cycles, cases where a cycle has yielded low-quality embryos, or during transfers in frozen cycles.
Endometrial scratching
It is a controlled endometrial injury; deliberate damage done to the endometrium in order to increase its receptivity and thus facilitate embryonic implantation.
Endometrial scratching is used for patients who, despite having a normal ovarian reserve and high-quality embryos during IVF, have not achieved implantation.



Atosiban infusion
As an antagonist of oxytocin and vasopressin receptors, it reduces uterine contractile activity and lowers intrauterine prostaglandin secretion, improving uterine blood flow and increasing the chances of successful embryo implantation.
Intravenous atosiban infusions are recommended for patients with diagnosed excessive uterine contractile activity, as well as for those who, despite a normal ovarian reserve and high-quality embryos during IVF, have not achieved implantation.
IVF procedure costs
Stage I: Carrying out the necessary examinations
| Compulsory women’s testing package required for assisted reproduction procedures – Anti-HIV – HBs Ag – Anti-HBc – Anti-HCV – VDRL – Toxoplasmosis IgG, IgM – Chlamydia PCR test – Cytology test – Vaginal cleanliness test – CMV antibiody test – IgG, IgM – Rubella antibody test |
PLN 740 |
| Compulsory men’s testing package required for assisted reproduction procedures – Anti-HIV – HBs Ag – Anti-HBc – Anti-HCV – VDRL – Chlamydia PCR test – CMV antibiody test – IgG, IgM |
PLN 540 |
| Compulsory pre-anaesthesia testing package – Blood count – Sodium – Potassium – APTT – PT If no blood type result PLN +50 |
PLN 80 |
Stage II: Hormonal stimulation and monitoring of the patient’s cycle
| Consultation, incl. ultrasound scan, during stimulation |
PLN 200 |
| Consultation, incl. ultrasound scan, during stimulation – Gynecological endocrynology and fertility specialist |
PLN 250 |
| Estradiol (E2) (K99) |
PLN 48 |
| Progesterone (N55) |
PLN 48 |
Stage III: Ovarian puncture
| Ovarian puncture |
PLN 1 650 |
| Anaesthesia for surgery, anaesthetic consultation, post-operative anaesthetic care |
PLN 750 |
Stage IV and V: In vitro fertilisation and embryo culture
| IVF/ICSI/IMSI embryology procedures supervised by the Matcher IVF electronic witnessing safety system with Time-Lapse observation of embryo development (24/7 every 10 minutes) and EmbryoPrime AI support |
PLN 6 400 |
Stage VI: Embryo transfer
| Embryo transfer |
PLN 1 800 |
Stage VII: Freezing of other embryos
| Embryo / blastocyst vitrification Price for the first embryo / blastocyst – each additional PLN +350 |
PLN 950 |
Costs of additional techniques
| Additional charge for Fertile chip ICSI (sperm segregation by microfluidics) |
PLN 1 100 |
| Hyaluronate transfer medium surcharge (EmbryoGlue/UTM Transfer Medium) |
PLN 600 |
| Assited Hatching |
PLN 600 |
| Endometrial scratching (controlled endometrial injury) with ultrasound guidance in female patients with recurrent implantation failures |
PLN 550 |











